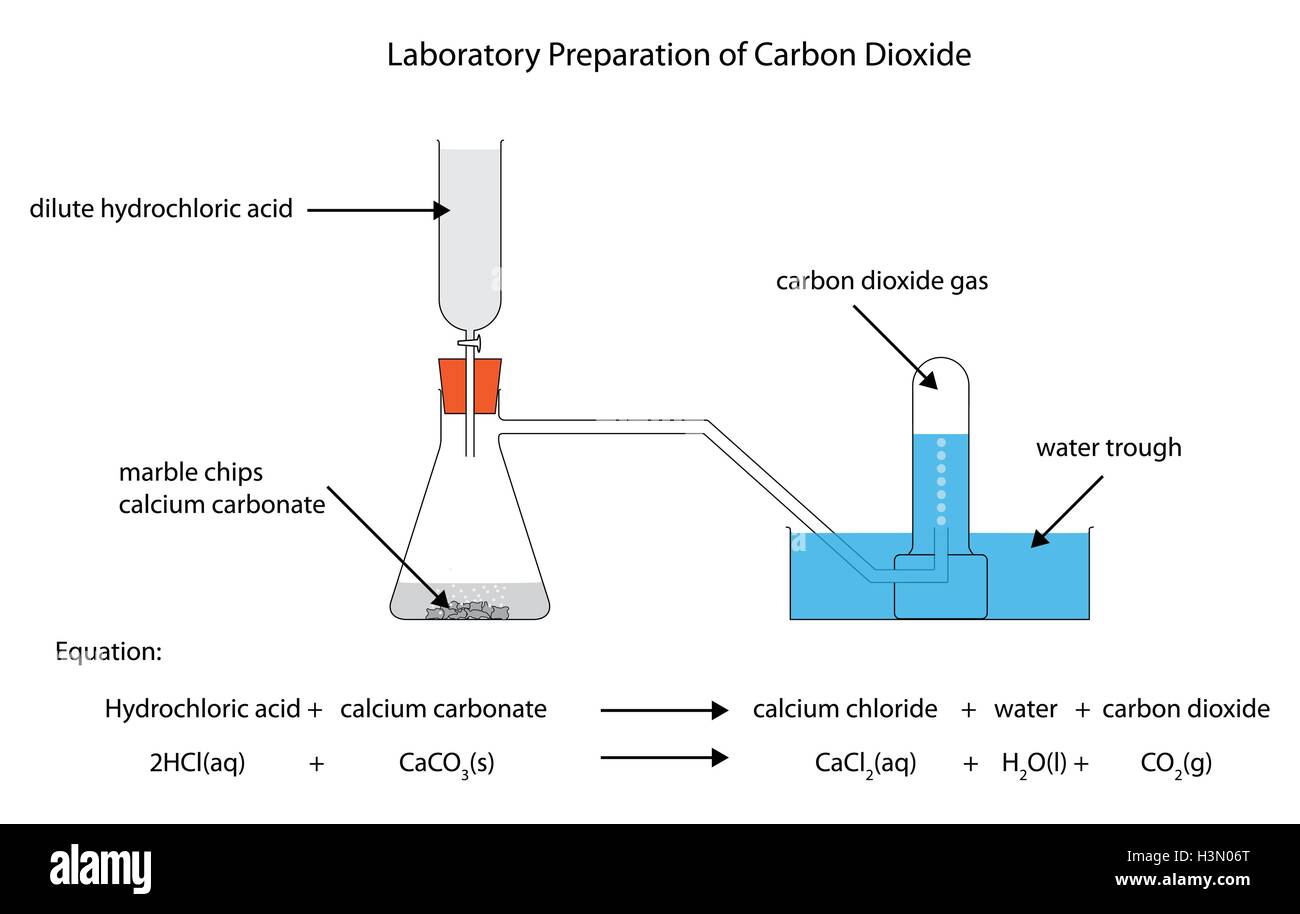
Caco3 2hcl h2o co2 this is the reaction we will be investigating.
Marble chips and hydrochloric acid graph.
A graph of decrease in mass against time is shown in fig.
A conical flask contains the marble chips hydrochloric acid and the water that will make the reaction.
The rate of this reaction can be changed by changing the size of the marble chips.
Task my task is to measure the rate of reaction between marble chips caco 3 and hydrochloric acid 2 hcl.
To investigate the effect of concentration on the reaction between marble chips and hydrochloric acid materials.
The variables that i shall be changing will be the concentration of hydrochloric acid and water.
Marble chips placed onto pieces of paper.
Place 40cm 3 of hydrochloric acid in an conical flask.
A stand to hold up the measuring cylinder.
Conical flask delivery tube bung measuring cylinder x 2 water trough water stopwatch marble.
An investigation into how changing one variable influences the rate of reaction between marble chips and dilute hydrochloric acid planning section when dilute hydrochloric acid reacts with marble chips the following reactions occurs.
A chemistry investigation to look at the rates of reaction between marble chips and hydrochloric acid.
Conical flask glass jam jars measuring cylinder stop clock watch with seconds stop watch app direct reading balance dilute hydrochloric acid cotton wool marble chips method.
Which of these statements explains what would.
Measured out 1ml of water in a 10ml measuring cylinder and placed into the test tube labelled 2.
Investigating the rate of reaction between marble chips calcium carbonate and hydrochloric acid aim.
0 5 g of magnesium ribbon and 0 5 g of magnesium powder are reacted with identical samples of hydrochloric acid.
Measured 5ml of hydrochloric acid in the 10ml measuring cylinders and placed into each beaker separately.
In the investigation i am going to find out how the surface area affects the rate of reaction by measuring the amount of gas produced and weight loss in a reaction between small large pieces of marble chips calcium carbonate and hydrochloric acid per minute.
A tube to connect the conical flask to the measuring cylinder.
Diagram plan the equipment i will be using for this experiment will be as follows.
If dilute hydrochloric acid is added to excess marble chips carbon dioxide is given off and the mass of reactants decreases.
C reaction of marble chips and acid.