Thus the presence of h 2 so 4 causes the concentration of h ions to increase dramatically and so the ph of the rainwater drops to harmful levels.
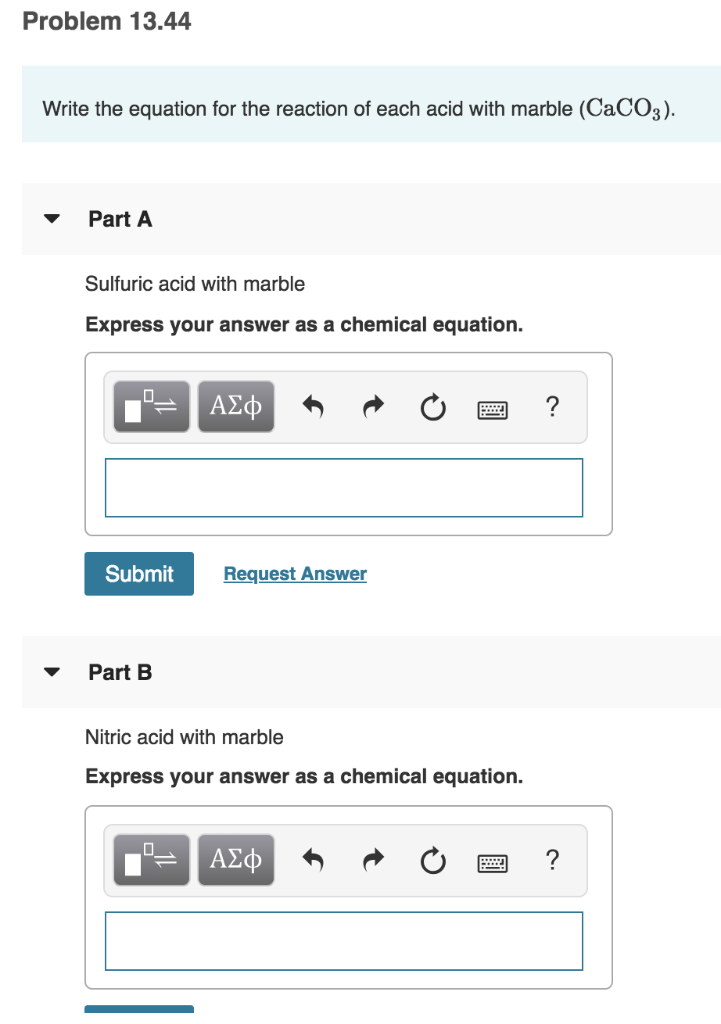
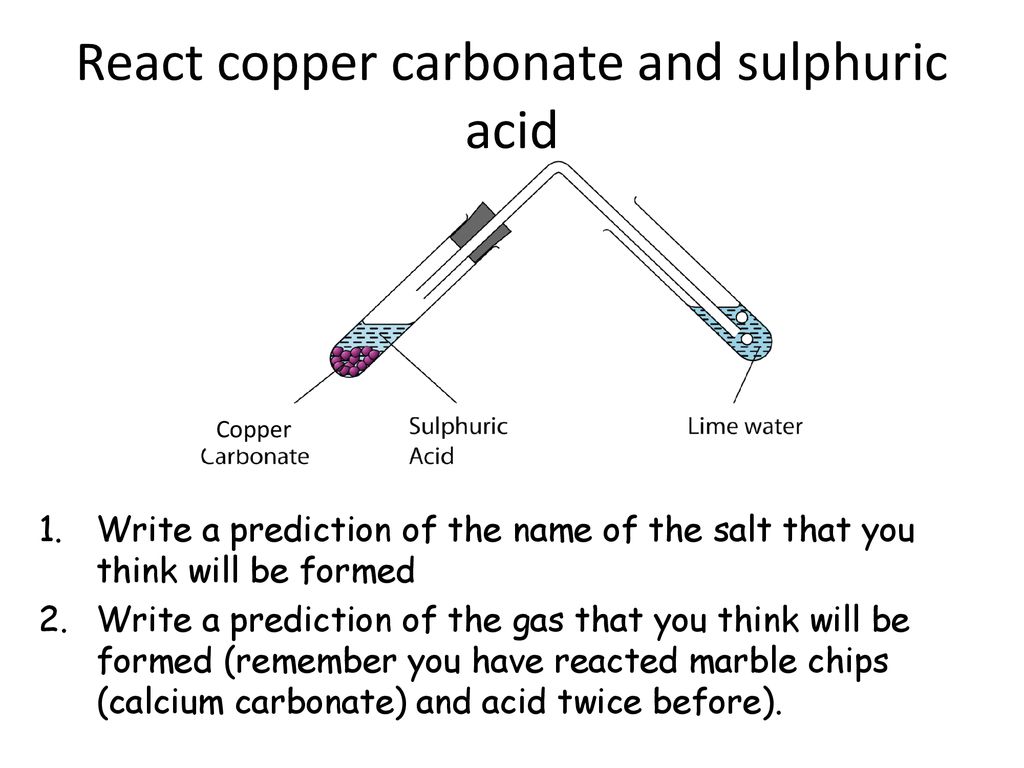
Marble and sulfuric acid reaction.
Sulfuric acid is a strong acid so it readily dissociates in water to give an h ion and an hso 4 ion equation 7.
Caco3 2hno3 ca no3 2 h2o co2 g i believe this reaction happens regardless of the concentration of nitric acid since.
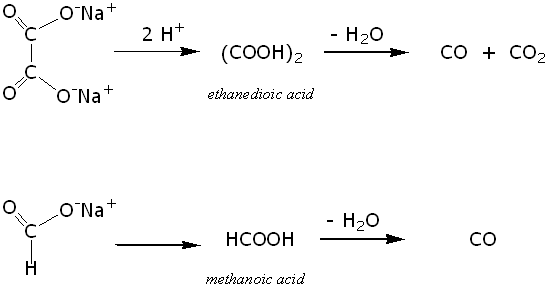
Create a dehydration reaction using sugar and sulfuric acid.
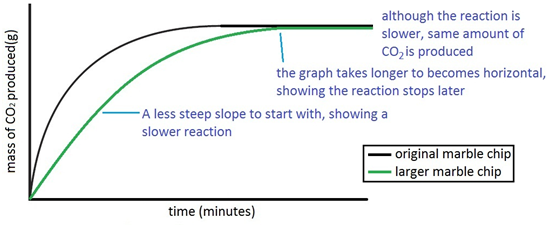
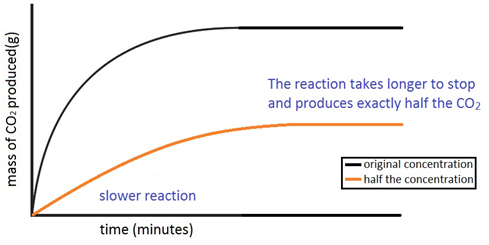
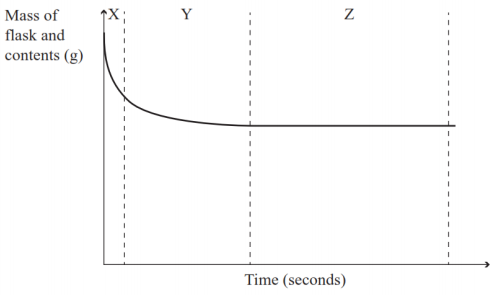
Marble chips react with dilute hydrochloric acid to produce carbon dioxide gas.
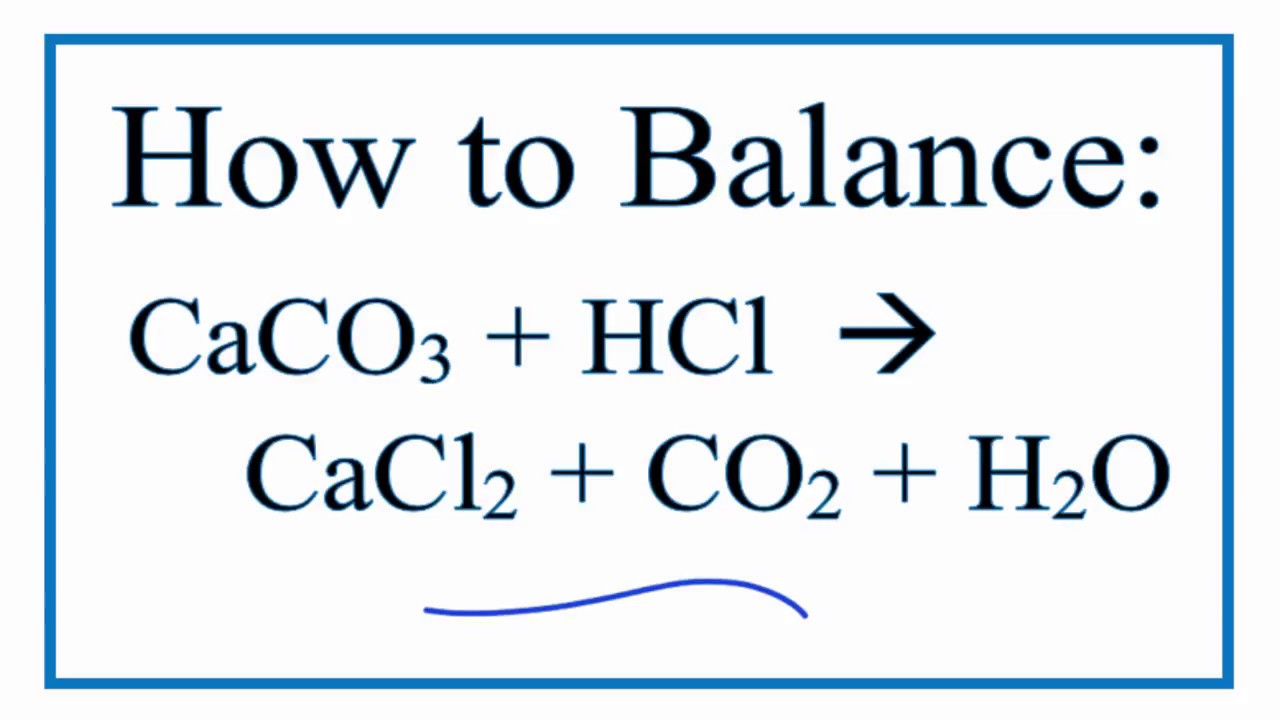
The balanced equation is.
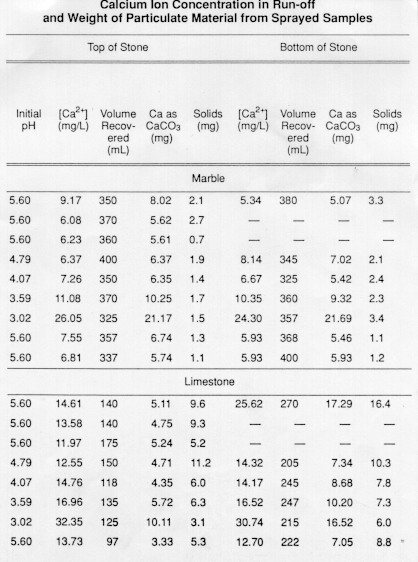
Stone surface material may be lost all over or only in spots that are more reactive.
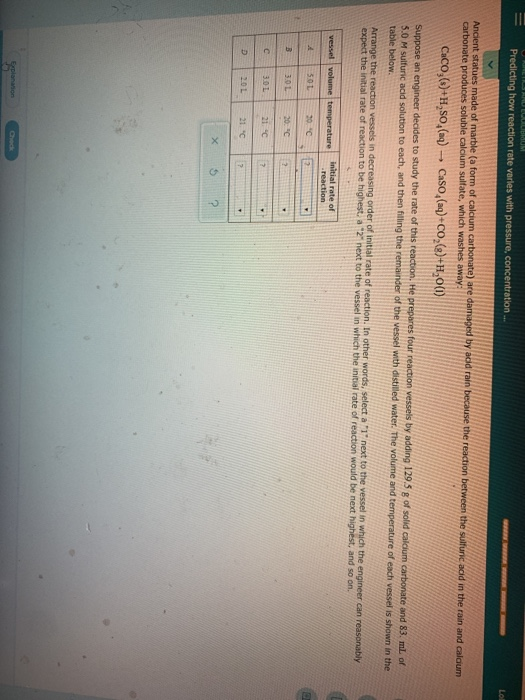
The reaction between sulfuric acid and calcium carbonate is somewhat similar to the reaction with sodium bicarbonate way carbon dioxide.
Sulphuric acid is a compound it is not sulfur dioxide and water the formula is h2so4sulphuric acid is the product of the chemical reaction that occurs when sulfur trioxide and water are mixed.
When acids react with carbonates such as calcium carbonate found in chalk limestone and marble a salt water and carbon dioxide are made.
The hso 4 ion may further dissociate to give h and so 4 2 equation 8.
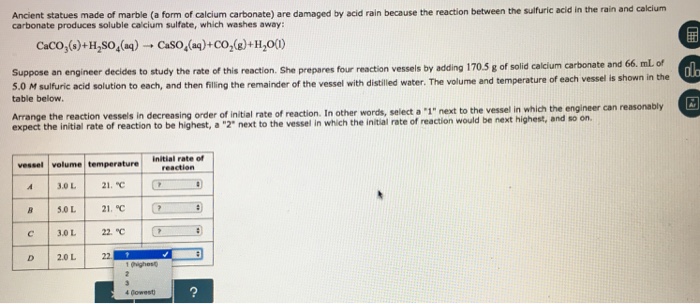
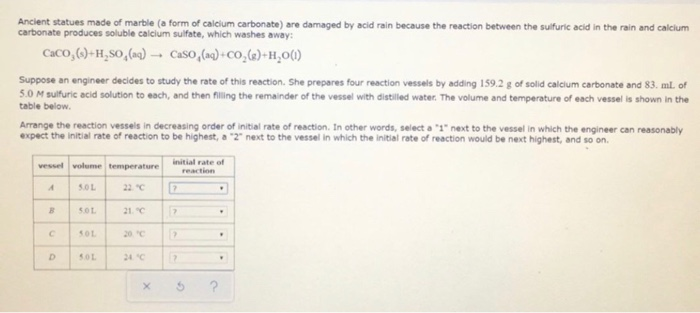
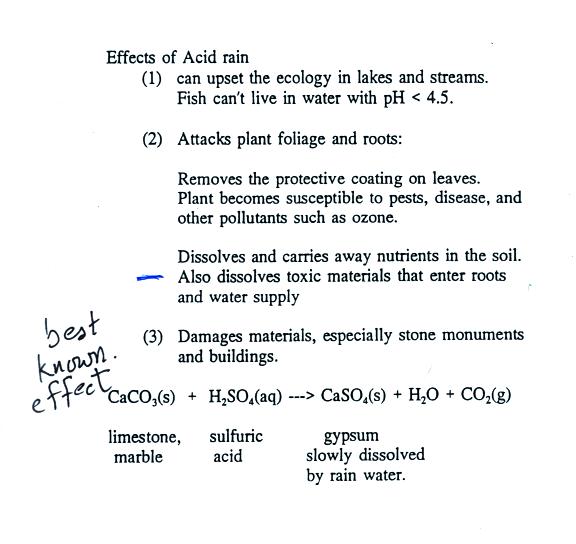
However sheltered areas on limestone and marble buildings and monuments show blackened crusts that have spalled peeled off in some places revealing crumbling stone beneath.
This black crust is primarily composed of gypsum a mineral that forms from the reaction between calcite water and sulfuric acid.
Dilute sulphuric acid is made to react with marble chips 2 see answers mahfoozfarhan4 mahfoozfarhan4 so when calcium carbonate reacts with sulphuric acid it forms water carbon dioxide and calcium sulphate.
The rate of this reaction can be changed by changing the size of the marble chips.
Sulfuric acid iron ii carbonate iron ii.
H2co3 aq h2o l co2 g so caco3 s h2so4 l h2o l co2 g caso4 s so when calcium.
Caco3 s h2so4 l h2co3 aq caso4 s 2.